Can the potential for metal-derived fuel be actualized?
In 2030, Mr. K picked up a container of fine black powder before going to work. Last night, on his way home, his automobile ran out of fuel, and the materials in this container will be used to refuel his car.
The substance he is carrying in his hand is steel powder, in nanometer (1/1,000,000,000 meter)-sized particles. Fuel made from metal may have only been thought to exist in stories, but in fact, research on ‘metal fuel’ is already in the process of becoming a reality.
Since thousands of years ago, mankind been able to use metal’s properties through burning. One example is the fireworks we use during celebrations, which are created by setting metal powder on fire mid-air. The concept of metal fuel is to use heat, light and explosive power, but for automobiles or power plants rather than just fireworks.
Metal actually has far more potential to act as a fuel than gases or liquids, as it produces much more heat from chemical reactions. For example, energy stored in 1L of LNG produces 22.2MJ (megajoules), but iron of the same volume produces 40.68MJ, and aluminum, 83.8MJ.

One of the well-known examples we use metal as fuel is for fireworks, which we ignite by setting fire on metal powder while it is in the air. Scientists want to use the heat and combustion energy generated during these types of reactions for running an automobile or a power plant.
There are two main ways to use metal as fuel for automobiles or power plants. The first is to set fire to metal when it is in the form of fine particles, and then have it react it with oxygen (4Al+3O2→2Al2O3). The enormous amount of heat generated can be used to operate an engine.
Another way is to combine metal powder with water. Recall the experiment during your elementary school science class when you poured water onto a glittering sample of aluminum. Soon after, the mixture would start boiling over. For this highly reactive outcome, the aluminum had reacted to hydroxyl ions immediately when water came into contact with it (2Al+6H2O→Al(OH)3+3H2).
This process generates hydrogen gas and heat. Hydrogen gas itself can be used as fuel (in the form of fuel cells), or, in other cases, the intense heat can operate an engine or turbine.

When the US army’s thermobaric weapon exploded, powerful shock waves were generated through an aluminum powder explosion, having the ability to destroy facilities or people.
Clean and convenient fuel – Overcoming its reluctant combustion with nanoscience

This is the biggest advantage of metal fuel. Neither of the two reaction formulas include carbon (C). This means it does not generate carbon dioxide, which is the main cause of climatic change. But this is not its only strength of; metal fuel is renewable. When you burn trees or coal, only unusable gray ashes remain, and other materials get carried away into the air. But when you burn metal fuel, you will get oxidized metal.
After separating oxygen from the oxidized metal through an additional process, you will get the same amount of metal you had before. You will never run out of fuel. Unlike renewable energy, like solar or wind power, you can also use it regardless of weather conditions. Another one of its great merits is that it can be imported and exported by cargo, with no need for installing a separate transmission network.
But using metal as fuel is easier said than done. First, the temperature needs to reach a certain level in order to set fire to the metal, which has an extremely high reactivity. Its high reactivity is, ironically, both its strength and its weakness as a fuel.
The principle of its low combustibility can be explained through this example: if you place pure metal in the air, it becomes rusty by reacting to oxygen and developing a thin layer (oxide coating) on its surface. You can burn metal when it comes into contact with an oxidizer like oxygen, but it interrupts the initial process.
This coating has a very high melting point normally: pure aluminum’s melting point is 660℃, but the oxide coating of aluminum has a melting point of around 2100℃. The burning temperature is not impossible to reach, but it is difficult to get it to that level due to stability and economic feasibility.
This is not the only problem. We still don’t know exactly which conditions produce the amount of firepower we need to safely produce fuel energy. We can burn very small metal particles easily, but the energy we obtain may be too little, or it may arouse the danger of explosion. However, if the particles are too big, they cannot be burnt easily. What we need to do technologically is to figure out the optimal particle size, mixture ratio, and content.
Another obstacle that inhibits the commercialization of metal fuel is that we don’t have the proper technology to stably supply it as needed. For example, when you drive a car, you can always start or stop the engine and drive to the desired speed at any time. This is possible because of the availability of the technology that exactly supplies fuel to the engine as needed.
Gas fuel can easily be controlled by a valve, and liquid fuel can be controlled with a spray. But in metal fuel, the amount is hard to be controlled due to cohesion, a unique property of its powdered form. Cohesion can be explained through imagining flour or powdered sugar forming a cluster. This is the real reason why previous plans attempted by many research institutes in order to develop an automobile steel have ended unsuccessfully.
Favorable conditions for producing electricity
Fortunately, however, several alternatives have been discovered as a result from foundational research on metal fuel. For example, you can easily burn pure metal powder by coating its surface with material that has a low melting point, such as nickel.
Nickel has a lower ignition temperature than aluminum, and reactions between metals generates heat, which in turn burns aluminum faster. (This metal reaction refers to the oxidation-reduction reactions that occur when metals with different ionization tendencies contact each other and have their electrons transferred.)
Recently, a collaborative research team consisting of two professors, Jeffrey Bergthorson and Samuel Goroshin, from McGill University’s mechanical engineering department in Canada, which is considered the world’s leaders in metal fuel research, has succeeded in producing the power equivalent to fossil fuel-powered engines with a heat engine using metal fuel they directly developed.
In the paper they published the ‘Applied Energy’ journal, the research team stated, “The first candidate that can be utilized for this purpose is steel”, and also that “Steel powder produced by steel manufacturers or chemical or electronic industries in the world can be used as fuel for automobiles.”
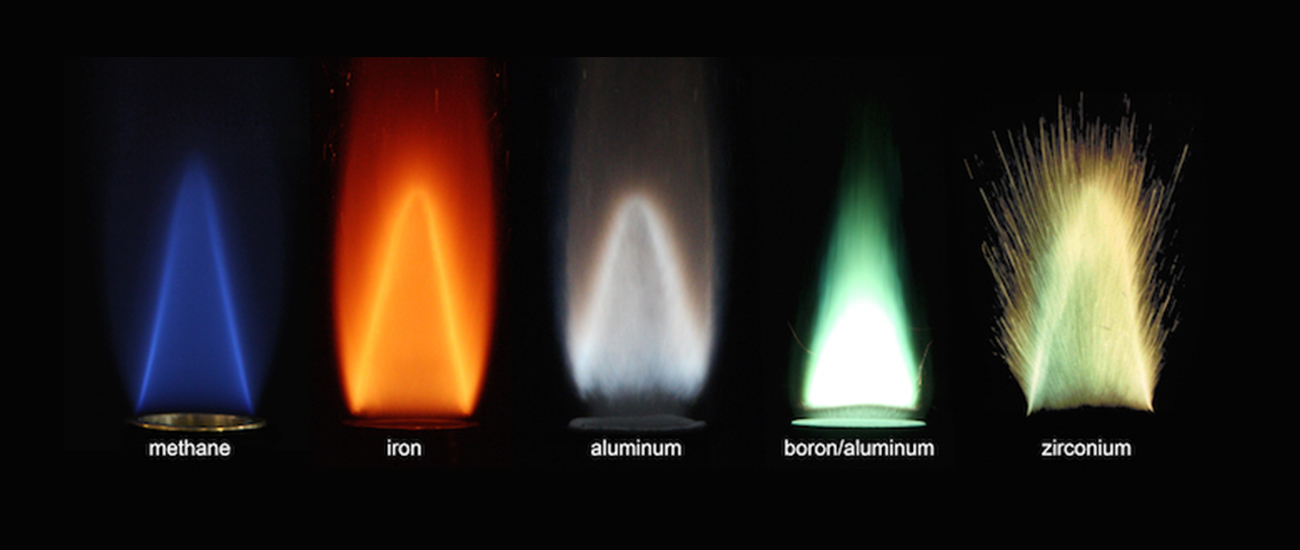
Results from experimenting with the combustion properties of various metals. Foundational research on metal fuel has finally reached its final step, and scientists are now researching into applying metal fuel to sources such as power plants.ⓒProf. Jeffrey Bergthorson of the mechanical engineering department at McGill University, Canada.

The concept map of an engine using metal fuel developed by Professors Jeffery Bergtorson and Samuel Goroshin of the mechanical engineering department at McGill University, Canada. The heat generated by this system is expected to be used to run an automobile or a home electricity generation system.ⓒSamuel Goroshin & Jeffrey Bergthorson (“Applied Energy” Journal)
Can we really have a car powered by steel in the future? Since metal is still more expensive than other fuel materials, it may not be able to replace every type of fuel. But in particular conditions where there is no oxygen, which is the most commonly used oxidizer, it may be successfully utilized to make electricity. For example, the metal powder in weapons from a supercavitation underwater vehicle can react to water and obtain its driving force. If we can develop technology to control this reaction, we may be able to get a new engine paradigm for a vessel that obtains its driving force by reacting metal powder to seawater.
Written by DongA Science reporter Ahyoung Woo
The opinions expressed in this POSCO Report piece are the author’s own and do not necessarily reflect the views of POSCO.

Celebrate the Radiant Science Behind Colorful Fireworks
