Firework shows are well-loved light performances that embody the festival spirit. Beginning with Canada Day (July 1) followed by Independence Day in the US (July 4), Bastille Day in France (July 14) and the Sumida River Firework Festival in Japan (July 30), this month is the prime time for celebratory fireworks around the world.
The beautiful fireworks at these events light up the sky in all shapes and colors, mesmerizing onlookers of all ages. However, there’s a whole lot more to pyrotechnic displays than their eye-catching aesthetics.
An Age-Old Tradition

Fireworks originated in ancient China in the form of firecrackers—explosives that were created by burning black powder inside bamboo stalks. Since then, firecrackers have become cultural symbols and are believed to be capable of warding off evil spirits thanks to their explosive sounds.
However, the colorful aerial firework we are most familiar with comes from Italy. Italian pyrotechnicians were the first to blend metal colorants and pyrotechnical mechanisms together, creating both visual and auditory entertainment for spectators.
Making Sparks Fly
There are at least five essential ingredients needed to produce pyrotechnical displays: metal colorants to produce various colors, fuel to detonate, an oxidizing agent to provide oxygen for fuel combustion, a chlorine compound to enhance colors and a binding agent to hold the compounds together.
Among its parts, the aerial shell in particular contains black powder and “stars” that are comprised of metal salt colorants. The ancient Chinese black powder formula used as fuel consists of potassium nitrate, charcoal and sulfur. When this formula ignites, it releases hot gases that have enough power to launch the firework and detonate the colorful stars high in the sky.
A Metal for Every Color

The beautiful colors that aerial fireworks paint across the sky vary depending on their composition. Made of up finely ground metals, these compounds, also called pyrotechnic colorants, determine the color, brightness and intensity of the light produced.
The colorants in fireworks are essentially metal salts that burn and release energy in the form of light. Each metal element corresponds to a particular wavelength of light when it burns, which is what humans see as visible electromagnetic light. These metal elements each emit specific amounts of energy that is characteristic of the element.
For example, a copper compound creates a burst of blue while calcium emits an orange tone. The chart below shows which metal element is used to produce each color of firework.
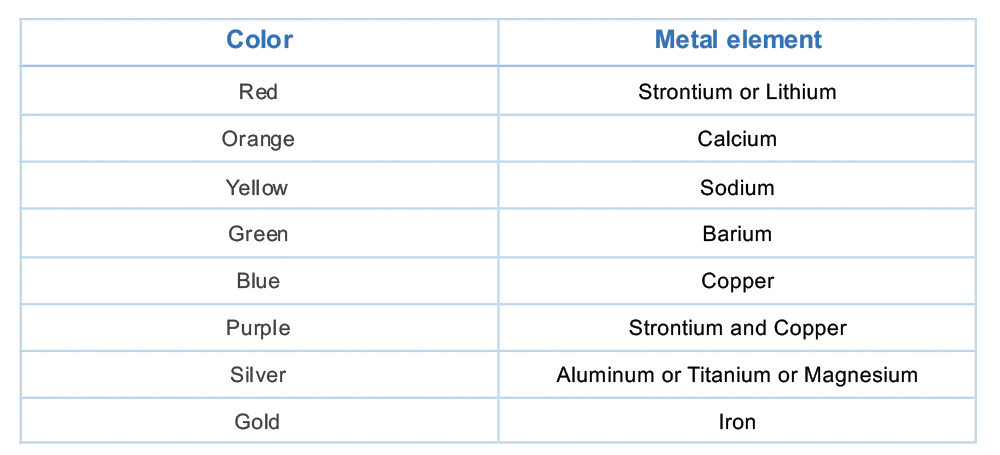
To obtain the most vivid display of color, the firework ingredients must be fresh and well put together. It is also essential for the final concoction to be carefully formulated, as the colors are extremely sensitive, even to trace amounts of impurities.
So, whether you’re celebrating Canada Day or have front row seats at the Sumida River Firework Festival, impress your friends and family with the color science of these amazing pyrotechnical displays. And don’t forget to enjoy the show!
